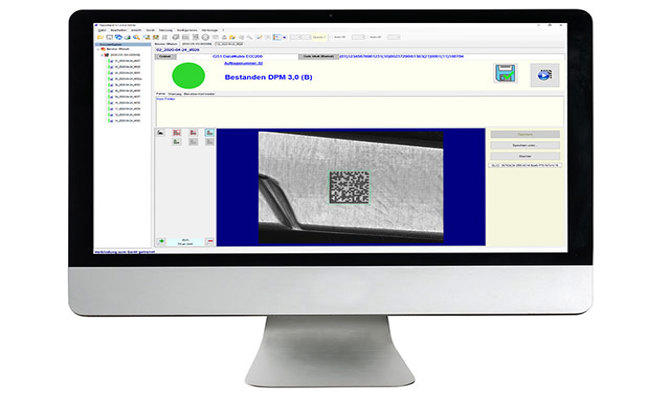
Unique coding and marking of medical devices to increase patient safety
The EU Medical Device Regulation (MDR) 2017/745 of the European Union obliges producers, distributors, importers and EU representatives of medical devices or their packaging to clearly mark their products as of May 26, 2021. Medical devices will thus be completely traceable from the manufacturer to the user and guarantee the patient safety required by the EU.
The standards and regulations passed in accordance with the Medical Device Regulation are equally binding for all players. To this end, the product identification required for the medical device is issued by one of the four allocation authorities – GS1, HIBCC, ICCBBA and Informationsstelle für Arzneispezialitäten.
From May 2022, all medical devices will be stored bit-by-bit in the EU-wide EUDAMED database with their master data and the “Unique Device Identification” (UDI).
The product identification consists of two components:
- Device Identifier (DI): a static code with approx. 20 data for manufacturer and product identification .
- Production Identifier (PI): variable data that serves traceability purposes, such as batch number, expiration date or serial number.
Classify medical devices correctly
Risk classes and categories
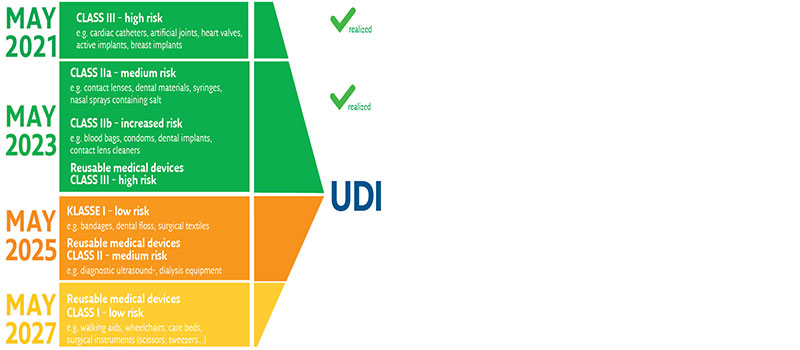
Medical devices are categorized and classified into risk classes, which are defined by the intended purpose in terms of the place and duration of use of the product.
There are e.g. products for single use or reusable medical devices, implants or others like software and devices.
The resulting risk classes then also specify the implementation deadlines in legislation.
Where must the UDI marking be applied?
Direct marking by ink, laser or label
The product identification must be applied either directly on the product, on primary packaging or on all higher packaging levels. According to the Medical Device Regulation the direct marking must be applied by ink, laser or label.
In addition, the code must be easily accessible and readable both in the warehouse and when the product is in use – and this throughout the entire life of the product.